I thought soap was supposed to be *clean*!

People usually assume soap gets rid of funky microbes that might grow on things, so I was very amused several months ago when I spotted something growing on top of the soap in one of the household hand-soap dispensers. As of today, it looks as pictured at left. That lumpy yellow and brown mass atop the the soap looked to me like some sort of soap-sodden mold, and have been saving the dispenser specifically in the hopes that someday I’d have a microscope and could take a look at it. Meanwhile, the mass spread, and slowly started releasing some kind of yellow pigment into the soap.
Incidentally, I kind of doubt this indicates some sort of failure on the part of the manufacturer of the soap. I don’t recall for certain, but I think I may have opened the dispenser at one point to transfer some of the soap to another nearly-empty dispenser. When the mass started growing originally, it was a single spot, which suggests a single spore or speck of dust floating in and landing on the surface. Hey, it happens. Anyway, I’ve therefore blanked out the name of the manufacturer since I don’t think they really have anything to do with this.
This mysterious growth upon my soap remained mysterious until today. Thanks to the Minister of Domestic Affairs and VWR (who managed to find me a really good deal), I finally got to actually get a close look at that lumpy mass. Meet my new friend Minnie (pictured at right). I could gaze into those eyes for hours. I couldn’t afford a darkfield condenser, and I sure as heck couldn’t afford to upgrade to phase-contrast gear, but I can add either one later if the opportunity presents itself. I also can’t afford the overpriced proprietary digital camera attachments either, though working around that is a whole other project. Until I identify an affordable model that plays well with Linux or work out how to modify a webcam into an ocular attachment,
I’ll have to settle for a trick…
It turns out if you take a digital camera and set it for close-up photos, you can actually stick the camera lens right up to the eyepiece and often get a serviceable picture.. Now, I had to subject the pictures I got today to moderately heavy processing to bring out the detail a bit better, but at least part of that is just me working on learning how to optimize the camera settings for this kind of use.
Equipped with some surplus slides and cover-slips donated by a kind professor who had some extra packages, I opened up the soap container and smeared a little of the yellow crud on a couple of them. One I just slapped a coverslip on for direct observation – the other I smeared over a slide and let dry with the intention of staining using the tiny, previously-unused vial of methylene blue left over from a very old plastic toy microscope. While the latter dried, I took a look at the wet mount hoping to finally see the mold mycelia that I had been expecting…
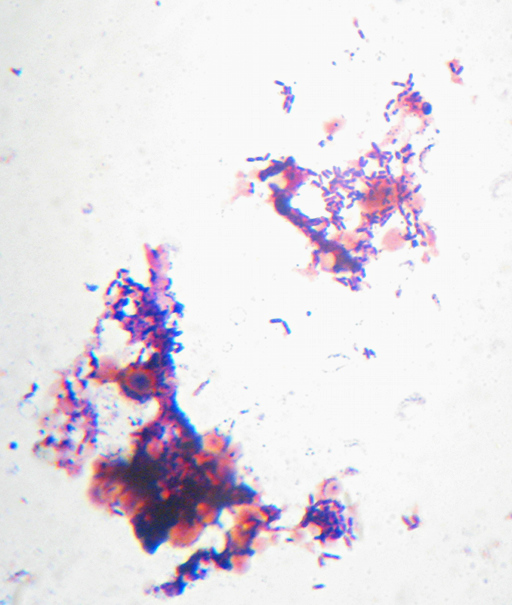
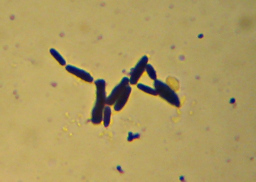
There wasn’t enough contrast to bother trying to get a photo, but it was obvious at 400x that what I was looking at was bacteria, not mold. Nerdly joy at learning something by looking in the microscope that I wouldn’t have otherwise known ensued, along with happiness as I realized this meant I had a perfect excuse to dig out my recent shipment from the Maker Shed – materials for doing a “Gram Stain”. Incidentally, the “Maker Shed” had the supplies on the way to me within hours of my ordering it, and they have lots and lots of cool stuff. I highly recommend it. Anyway, I got to do a “Gram Stain” for the first time in a couple of years (and the first time ever outside of a school lab). Want to see?
Mystery Microbe, I see you!
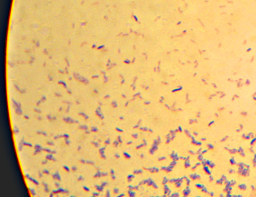
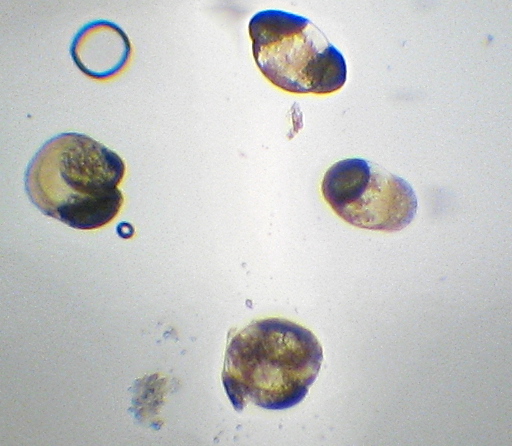
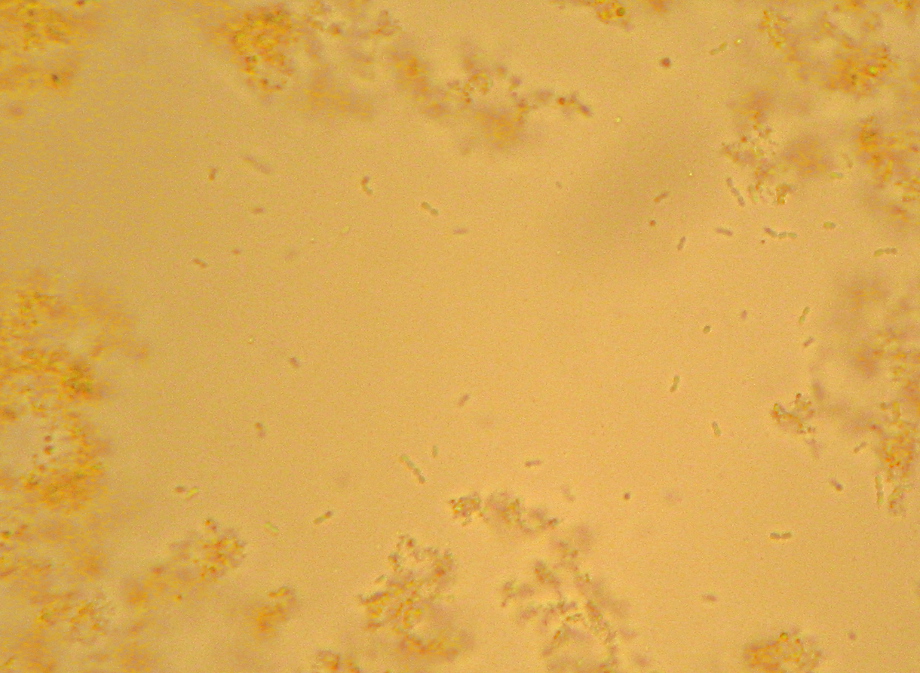
Here it is – the nasty yellow goo that infected my bottle of hand-soap. My staining technique was a little off since I’m out of practice – the way I interpret the results is that what I’ve got here is neither a member of the Firmicutes (i.e. “Gram positive”) nor – probably – Actinobacteria. I really can’t guess at more than that, though. I think the few “Gram-positive”-looking cells there are artifacts of insufficient decolorization. I know I still had a surplus of the purple “Crystal Violet” stain still on the slide at the end. (How did I know? I’ll show you at the end…). The irregular bluish bits towards the bottom are, I believe, just bits of stuff from the soap itself.
Meanwhile, this pretty much satisfies my curiousity about the Mystery Soap-Infecting Microbe. There’s certainly a lot more I could investigate, but my developing Hillbilly Biotech lab is really intended to support my interest in intentional food microbiology and perhaps evenutally some small-scale non-food industrial microbiology. I have some remaining curiousity about the yellow pigment and whether or not it might be useful for something, but I’m doubting there is any food or beverage I might want to grow this stuff in and therefore don’t have much use for it. Still, I’ll keep the bottle around for a while before I throw it out in case I think of something fun to do with it. If I end up being really interested in the identity of the bug growing on it, I should be able to find a liquid that I can grow a big mess of it in, then run it through a simple DNA extraction process. Then all I need to do is find someone who can supply PCR primers, a thermocycler, and sequencing services cheap. It might sound like I’m being facetious, but I wouldn’t be surprised these days if I manage to find somewhere that’d do it for $20/sample or less. I may eventually do this will the Mystery Soap Bug anyway, since I hope to be running through this process with cultures of sourdough, yogurt, cheese, vinegar, and brewing microbes that I develop myself. For now, though, it’s just nice to be playing with microbiology equipment again. And now fully independently! Wheeeeeeee!!!!!!
Yes, I’m a nerd. And proud of it!
What’s next?
Now that I finally have a microscope, I no longer have any excuse for not getting to work on the rest of my Hillbilly Biotech lab. Just this weekend I was pricing out Hillbilly Autoclaves. I picked up a cheap air pump and air stone
for potentially building an aerobic bubble-column fermenter (for quick growth of yeast starters or a working model of a “Fring’s Acetator®”-style vinegar generator. I still want to build an ozone generator for sanitization and to get a pH meter. I’d like to also get my hands on some wheat, barley, and rye seeds to sanitize, sprout, and grow here as the first stage of developing a truly local sourdough culture, plus arrange to have several pounds of plain flour irradiated to sterilize it.
I’m also like summer to be over. Yes, I’m writing this in Winter, but it’s not until later in the summer to autumn that locally-grown fruits will start becoming available, and locally grown fruits ought to be an ideal source of local brewing and baking yeasts and bacteria. Finally, I’d like to find a wealthy patron (or matron, I’m no sexist…) who would sponsor me so I could just pursue food-microbe bioprospecting and research full-time…
Oh, yes, and I need to get around to finishing Episode 4 of my little podcast project, especially since episode 4’s topic is a fundamental microbiology technique.
Comments welcome below – thanks for reading!
Oh, and as a reward for getting all the way to the end, here’s a picture that I thought was pretty – crystals of “Crystal Violet” and iodine. I told you I had too much left on the slide…
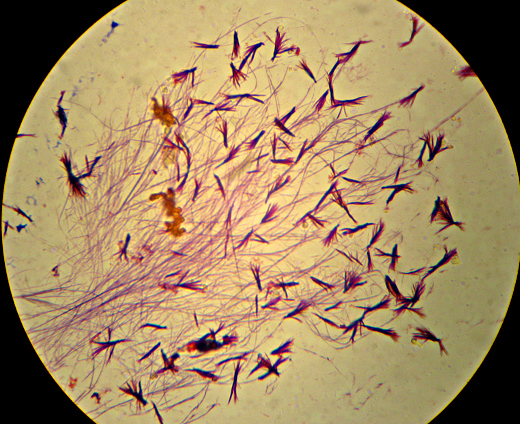
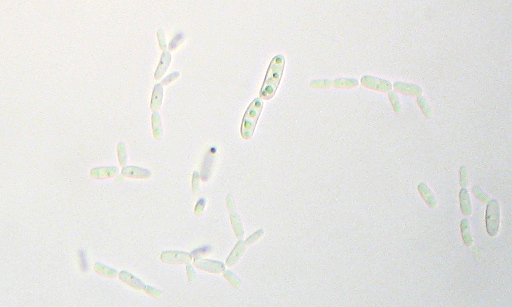
 I’m a bit puzzled that I don’t see anything that looks much like bacteria in here. “
I’m a bit puzzled that I don’t see anything that looks much like bacteria in here. “ This unassuming little flower just happened to be conveniently near here. As far as I can tell, it’s a “Lyreleaf Sage” (Salvia lyrata). I plucked a flowering section and dropped it into a container of my special selective media a couple of days ago. It’s starting to get a little
This unassuming little flower just happened to be conveniently near here. As far as I can tell, it’s a “Lyreleaf Sage” (Salvia lyrata). I plucked a flowering section and dropped it into a container of my special selective media a couple of days ago. It’s starting to get a little