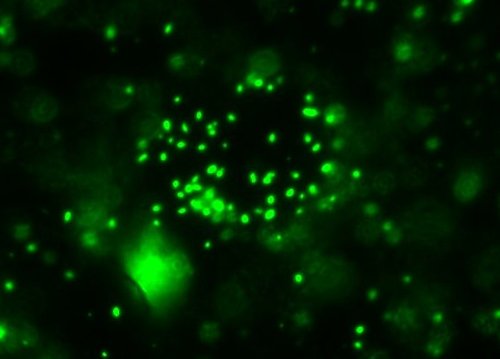
Which I think I shall designate as Ulysses (the Ubiquitous). This sample came from what appeared to be an individual colony on an agar plate made with ~8% salinity diluted Great Salt Lake water. I tried to ‘pick’ a colony with the tip of a hypodermic and ‘poke’ it into the anaerobic tubes. Guess it may have worked, at least with a few cells. (This was done 6 months ago, so if I even got only a few cells over, it’s had plenty of time to grow…) The picture’s a little overexposed (and I tweaked the contrast) though…
In any case, there’s a metric [pants]load of sulfate in the water. That means there’s PLENTY of it there for the microbes to breathe and turn into sulfide. No need for any of them to breathe the iron then.
I haven’t given up on that totally though – apparently molybdates can inhibit sulfate respiration, or so I’m told, so I can try making up a solution with some sodium molybdate added. Alternatively, now that I know the Great Salt Lake is very nearly just “concentrated seawater”, I can always make up some “artificial” sea salt, but substitute something else for most of the sulfate and see if things still grow on the iron oxides. That’s likely my next step as a last try to see if any of my Babies can actually breathe the iron. (This is in addition to trying to purify some DNA from Ulysses there, since I think that one is a pure culture.