Where Was I, Episode 3 – “It’s a Small World After All” (now updated!)
Study is sucking up most of my time, but that doesn’t mean I never go anywhere any more. Recently, I went to the “Edson Fichter Nature Area”.

Yeah, that place. But look closer.

No, no, I mean go
inside and look closer. Down there, by the river.

No, no, go closer.

CLOSER!!!
There, that’s a closer look. Incidentally, as you can see, the trick of setting the camera for close-up photos and sticking the little lens opening into the eyepiece of a microscope actually does work. Conceivably, then, the same trick ought to work with any kind of scope, which I’ll have to keep in mind for the next time we go to visit the Bruneau Observatory. But I digress…



I have no idea what all these neat-looking funky things are. Well, not besides the fact that they’re little
animalcules with
cilia and stuff swimming around and that they’re pretty big as far as microbes go.See, I’m not really into
eukaryotes. Needlessly complicated, I say. I’m interested in the
cool stuff.
Useful stuff.
No ponderous huge protozoans for me, I’m hunting Our Friends, the Bacteria.
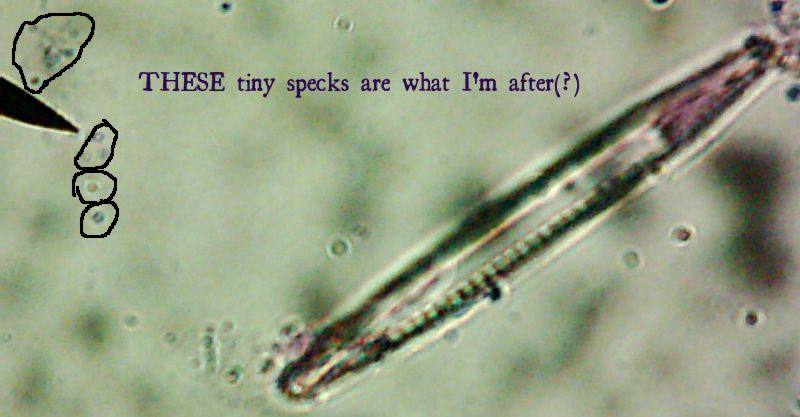
See them in there? No, no, you have to look even closer. What do you mean you still can’t see them?

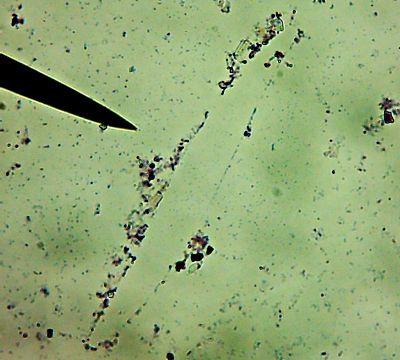
All right then, here, let me dry the sample out and
heat fix it, then I’ll
Gram Stain them for you.Wow. I guess soft, wet, squishy protozoal cell membranes don’t like being heat-fixed, do they? Ouch.
I’m not sure how much of that is bacteria and how much of that is exploded protozoan guts.
Let me look around elsewhere on the slide…
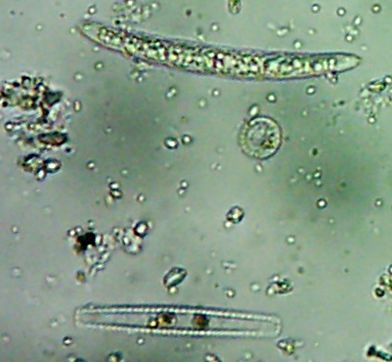
 Okay, technically at least some of these things are diatoms rather than “protozoa“.
Okay, technically at least some of these things are diatoms rather than “protozoa“.
Kinda neat and creepy at the same time, ain’t it?
Okay, enough staring at hollow shells of dead things, let me see if I can find some bacteria…
 Now, months later, I’m still not sure how much of the debris on those original slides was bacteria and how much was just crud from the samples. I did, however, get pictures of the 10 isolates that I wanted to try to identify (and of which, as you know from my entry on Willy Bacillus, I so far only know one…)
Now, months later, I’m still not sure how much of the debris on those original slides was bacteria and how much was just crud from the samples. I did, however, get pictures of the 10 isolates that I wanted to try to identify (and of which, as you know from my entry on Willy Bacillus, I so far only know one…)
Of the variety of isolates we got, I pulled out seven that showed some degree of fluorescence under my homebrew UV-LED flashlight, one that was growing in media containing 10mM of a zinc salt, one that was growing in scalding-hot conditions (55°C – which is about the temperature that “melts” collagen into gelatin and makes that tough stew-meat moist and tender), and of course, Willy Bacillus who grew in 10% salt.
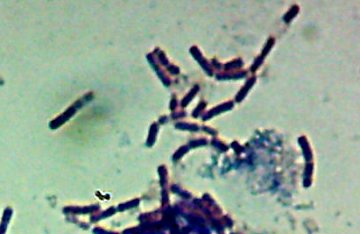
Pyotr, Paul, Olga, Ursula, Peggy, Penny, and Ada (the fluorescent bacteria) and Castor (the zinc-tolerant bacterium) all looked more or less like this when I gram-stained them:
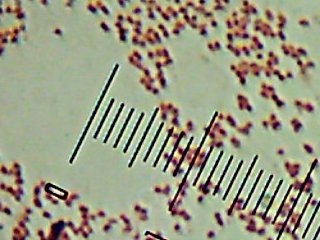
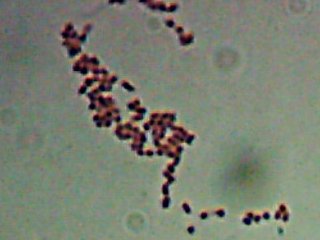
I think the Gram-biguous effect is more because of my technique than the bacteria. I believe these are all probably really gram-negative.
There’s a portrait of Willy Bacillus in the other post. Horace was the other interesting-looking culture:
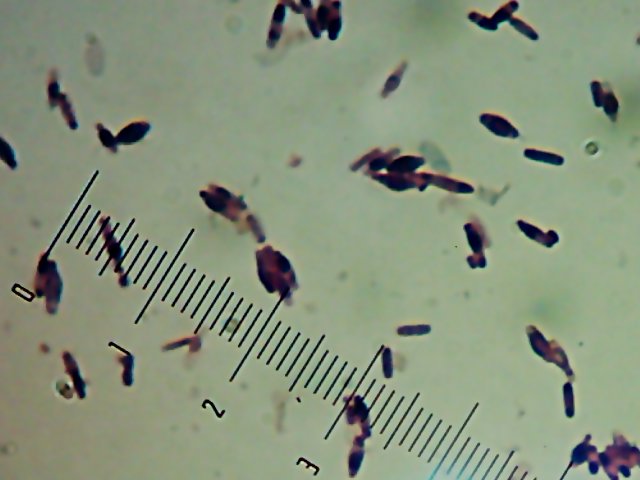
I’m not sure what’s causing the funny spore-like spots or the bulging of the cells. It might be normal, or it might be an effect of the heat-fixing or being in the “cold” (room temperature) or something. I’d be interested to find out what it is.
More fun facts:
- Olga and Ursula both grew in the ‘fridge (4°C)
- Ada’s fluorescence was different from the others – it was a dim blue rather than a bright blue-green.
- Ada also seemed to grow fine on media without nitrogen in it.
- Peggy was also releasing a green substance into the media.
- The quick-and-dirty metabolic tests we did (with Enterotubes™ did seem to imply differences between these different isolates.
One lesson driven home this semester was that you just can’t identify bacteria based on simple things like what they look like or a few simple observable traits. However, I still suspect there’s a good chance that everyone but Ada (and maybe her, too) is a Pseudomonas of some flavor, and all of them in any case are probably Gamma-proteobacteria, just based on the fact that when I went looking for information on fluorescent bacteria, nearly everything I found was about species of Pseudomonas, and the ones that weren’t were all still Gamma-proteobacteria. Plus, the media and techniques we used (and possibly the site itself) seemed to heavily favor the isolation of gamma-proteobacteria. If anyone’s bored enough to care, I can post something more about where we got the bacteria and why I wonder if the site had an influence on the heavily slanted taxonomy of our isolates this semester.
And that’s Where I Was.Where Will I Be next? Will my next post be something exciting, interesting, and insightful? Or will I just bore the holy living crap out of everyone with a couple of quickly-crammed-together trips with a couple of minimally interesting pictures? Even I don’t know! Tune in next time and find out!
Meanwhile, as always, questions, comments, and suggestions are welcome.
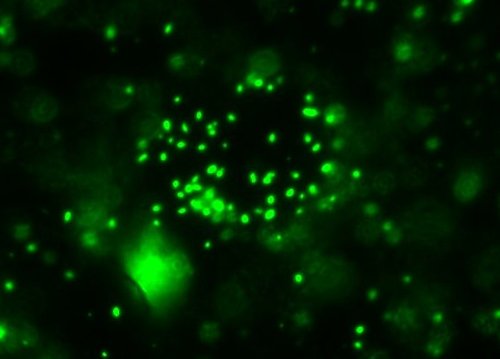
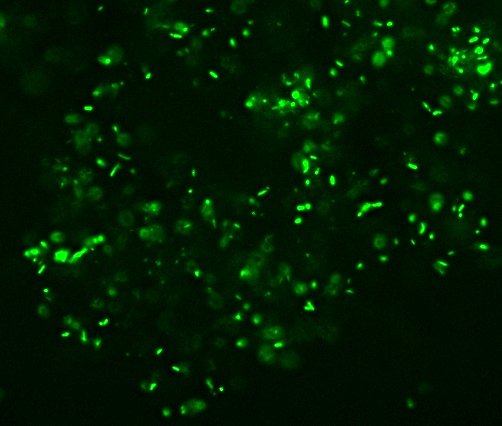









 All right then, here, let me dry the sample out and heat fix it, then I’ll Gram Stain them for you.Wow. I guess soft, wet, squishy protozoal cell membranes don’t like being heat-fixed, do they? Ouch.
All right then, here, let me dry the sample out and heat fix it, then I’ll Gram Stain them for you.Wow. I guess soft, wet, squishy protozoal cell membranes don’t like being heat-fixed, do they? Ouch.
 Okay, technically at least some of these things are diatoms rather than “protozoa“.
Okay, technically at least some of these things are diatoms rather than “protozoa“. Now, months later, I’m still not sure how much of the debris on those original slides was bacteria and how much was just crud from the samples. I did, however, get pictures of the 10 isolates that I wanted to try to identify (and of which, as you know from
Now, months later, I’m still not sure how much of the debris on those original slides was bacteria and how much was just crud from the samples. I did, however, get pictures of the 10 isolates that I wanted to try to identify (and of which, as you know from