Hello again, girls and boys and whatever else may be out there reading. It’s time once again for this blog’s contribution to The Giant’s Shoulders (hosted this time over at “Second Order Approximation“). This month I’m looking at something even smaller than usual. Inspired by my new job in the biochemistry department where they seem to do a substantial amount of X-ray crystallography (which I do not – yet – understand as well as I’d like), I thought I’d drop all the way down to the molecular level and talk about a fundamental discovery that helped make modern molecular microbiology possible: the structure of DNA.
Yes, it was over a half-century ago that an accomplished scientist, working with X-ray images produced from crystallized nucleic acids, published the first proposed structure for for them – the famous Triple Helix.
“Hey, Stupid!”, I hear you cry, “It’s DOUBLE Helix. Everybody knows that!” Well, yeah, now they do. But back then we had this:
Are you calling the late Dr. Linus Pauling a liar? The man won two freakin’ Nobel Prizes, dagnabbit! Okay, so one of them was merely a “Peace” prize, but still – that’s more than you ever did! So there!
Actually, Pauling did obviously turn out to be wrong here, and this paper was a serious disappointment to me. Not because it turned out to be wrong – hey, this is Real Science, and in Real Science things are often found to be wrong. They get corrected quickly and we move on with our scientific foundation that much stronger. That’s how it works. What disappointed me is that I had hoped that I’d be able to feed my ego by taking all the new scientific knowledge that has since slipped down into common undergraduate education in the last 50+ years, and then use that to point out the now-obviously-dumb assumptions in the paper. I could then smugly pretend that this made me smarter than Dr. Linus Pauling and therefore some kind of Super Genius® (just like Wile E. Coyote!).
Perhaps someone with a stronger modern education in physical chemistry might succeed here, but sadly on closer reading the paper’s conclusions seem frustratingly plausible to me. The Grossly Oversimplified Science™ version of this paper works something like this:
Pauling and Corey knew a few things from the existing knowledge. It was known that each individual piece of a nucleic acid was made of three parts – a phosphate, a sugar made from 5 carbon atoms (“ribose” in the case of ribonucleic acid [RNA], or the slightly different “deoxyribose” in the case of DNA), and a part that could vary between four different structures (one of two different “purine” or two different “pyrimidine” components). The molecular structures of these bits had been worked out before, and they seem to have known where the three components connected to each other to form each unit (or “residue”). They also had electron microscope photographs of individual strings of nucleic acid, from which Pauling and Corey concluded the molecules were long cylindrical things (not flattened ribbon-shaped things). They knew that proteins often ended up assembled into helices (coils, like a spring). And, finally, they had some X-ray diffraction images of nucleic acids to look at. Seeing a reflection in the X-ray imagery that suggested to them that the nucleic acid strand was made up of 3-residue chunks in some way, they proposed that each strand was actually three individual strings of nucleic acid residues wound around each other.
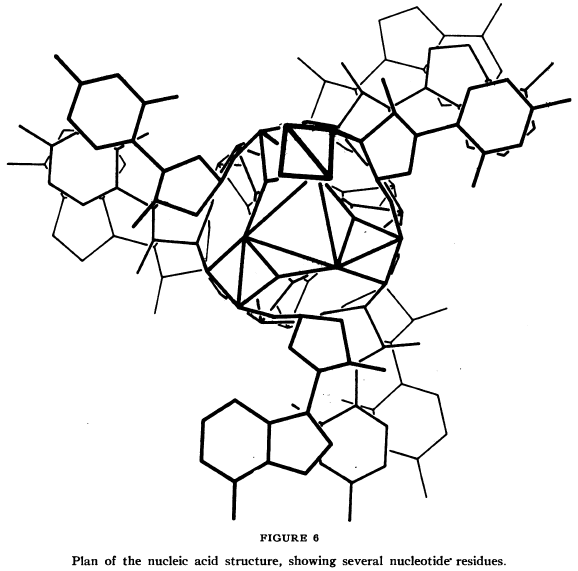 The latter seems to be the assumption upon which the rest of the paper’s proposed structure is based, and most of the paper deals with supporting evidence for why this could work.
The latter seems to be the assumption upon which the rest of the paper’s proposed structure is based, and most of the paper deals with supporting evidence for why this could work.
The only way they could get the components to fit together in a plausible way was to stick the phosphate parts together in the middle of the triple-helix. I thought I had him there at least – phosphate (PO4– is a single phosphorous atom surrounded by 4 oxygen atoms…and as long-time readers of this blog know, Oxygen Sucks (electrons). Whenever you cram a bunch of oxygen atoms together you normally get at least a partial negative electrical charge there from all the electrons spending so much time around them, and multiple negatively-charged bits will tend to repel each other. Wouldn’t that prevent the strands from getting near each other and twining together? How could a guy whose specialty was inter-atomic bonds not know this?
Naturally, having no consideration for my feelings, he even had a reasonable answer for this. Pauling and Corey cite a contemporary paper which showed an ammounium phosphate compound which had a structure including phosphates bonded together. Jerks.
So, thanks a lot, Dr. Pauling, for robbing me of a few minutes of smug superiority.
The paper’s proposed structure still turned out to be quite wrong, of course, when not long afterwards Watson and Crick got their hands on what was evidently much higher-quality X-ray imagery produced by the sadly under-appreciated Dr. Rosalind Franklin, and were able to work out the correct “Double Helix” structure. Still, one of the wonderful things about science is that even a wrong result retains educational value. Download the paper and check it out.
Any questions?…
3 thoughts on ““A Proposed Structure for the Nucleic Acids””