One more for the “classic papers” challenge:
Schaeffer AB, Fulton MD: “A Simplified Method of Staining Endospores”; 1933; Science; 77; pg 194
If you take a microbiology lab, this is the endospore staining technique (or “technic” as they used to spell it) that you’ll practice. This is a nice, simple, one-page paper. Alice B. Schaeffer and co-author Mac Donald Fulton describe a few of the other variations on endospore staining techniques, then describe how they’ve further simplified what they felt was previously the simplest one, described by a Mr. Wirtz in 1908.
“Endospores” are a sort of “escape pod” for certain specific kinds of bacteria. Unlike spores formed by yeasts and molds, these are not reproductive – each bacterium only produces one thick-coated spore, into which it shoves it’s genetic material and a few vital enzymes to get itself going again later when the spore finds itself in favorable conditions.
Since only a few kinds of bacteria produce these endospores, if you see endospores in your unknown bacterial culture it goes a long way towards helping to identify the bacterial species, so having a simple method for staining your bacteria so that endospores are obvious under a microscope is helpful. (Of course, these days most of us would rather just get a 16s rDNA sequence with PCR, but never mind that for now…)
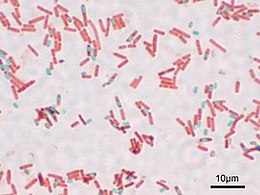 Evidently, Wirtz’s original method involved using Osmium Tetraoxide (“osmic acid”) to stick the bacteria to the slide before staining. Not only is that stuff poisonous, it’s also expensive. I found a site selling sealed glass ampoules containing 1 gram each of this stuff for $35.00 each. Schaeffer and Fulton’s method does away with this in favor of much cheaper and easier heat-fixing (just as is done with the Gram stain and others). They use the dye “Malachite Green” for the initial stain, and steam-heat the dye-covered bacterial slide a few times to sort of “cook” the dye into the thick-walled endospores if they are there. Rinsing then washes the dye out of everything but the endospores, and a light red dye (safranin) is added as a counterstain. The end result is that under the microscope you’ll see light-red bacteria. If any of them form endospores, you’ll be able to see them as smaller green dots – sometimes still bulging inside of bacterial cells, sometimes floating around freely having escaped from the now-empty bacterial cell.
Evidently, Wirtz’s original method involved using Osmium Tetraoxide (“osmic acid”) to stick the bacteria to the slide before staining. Not only is that stuff poisonous, it’s also expensive. I found a site selling sealed glass ampoules containing 1 gram each of this stuff for $35.00 each. Schaeffer and Fulton’s method does away with this in favor of much cheaper and easier heat-fixing (just as is done with the Gram stain and others). They use the dye “Malachite Green” for the initial stain, and steam-heat the dye-covered bacterial slide a few times to sort of “cook” the dye into the thick-walled endospores if they are there. Rinsing then washes the dye out of everything but the endospores, and a light red dye (safranin) is added as a counterstain. The end result is that under the microscope you’ll see light-red bacteria. If any of them form endospores, you’ll be able to see them as smaller green dots – sometimes still bulging inside of bacterial cells, sometimes floating around freely having escaped from the now-empty bacterial cell.
The “Schaeffer-Fulton Endospore Stain” is pretty easy to do, though the occasionally messy steambath part can be annoying. The method is pretty resistant to errors, so it’s not too hard to get good results even if you’ve never done it before.
Incidentally, you can buy Malachite Green at many pet stores – it’s still used as a treatment for “ick” (Ichthyophthirius infestation) in tropical fish.
Hmmm…still a couple of hours before it’s not longer May – Perhaps I can throw in one last post before time’s up…