Various yeasts of the genus Saccharomyces (particularly the “Baker’s Yeast” Saccharomyces cerevisiae) represent quite possibly the most important bit of intentional microbiology that we have. We eat and drink the little critters and their byproducts in more or less every human culture that I know of, and are now getting more seriously into burning them, too.
As I’ve mentioned before, gluttony is my second most favorite deadly sin, so bread and booze microbiology is naturally of interest to me. It seemed worthwhile to look into developing my own yeast (and bacteria…but that’s for another post) stocks to brew, vint, and bake with, so I did some poking around. I dug out my copy of Rog Leistad’s “Yeast Culturing for the Homebrewer”, Peter Duncan and Bryan Acton’s “Progressive Winemaking”, a number of internet sources, and finally some scientific papers. I know, I’m a nerd.
I have so far not found much of anything about isolating yeasts from scratch – virtually everything seems to assume that you will “buy” your yeast from somewhere else, and aside from scientific papers most assume that you’re only bothering to culture your own yeast to save money by stretching the sample you bought to brew several batches before buying more yeast from “the professionals” again. This annoys me.
Unfortunately, I’m still on the road and haven’t had time to directly embark on my culture project here. I’m also having a heck of a time tonight trying to come up with a way to make the process of isolating a pure culture sound interesting to anyone besides me. Here’s the extremely abbreviated version:
- Take something that’s got (in this case) yeast in it (sourdough starter, unfiltered beer, whatever)
- Make up some solidified yeast food: typically this is something like a mixture of sugar, predigested milk protein, and water, mixed with agar to solidify it, and with a small amount of acid added, since the acidity helps inhibit bacteria that might contaminate the yeast culture
- Take a tiny bit of the original stuff-with-yeast-in-it, and smear it thinly over the top of the solid medium.
- Cover the solid medium and put it somewhere warm for a while until you can see individual spots (“colonies”) of growth
- Take a bit of a single colony and put it in some sterile culture media.
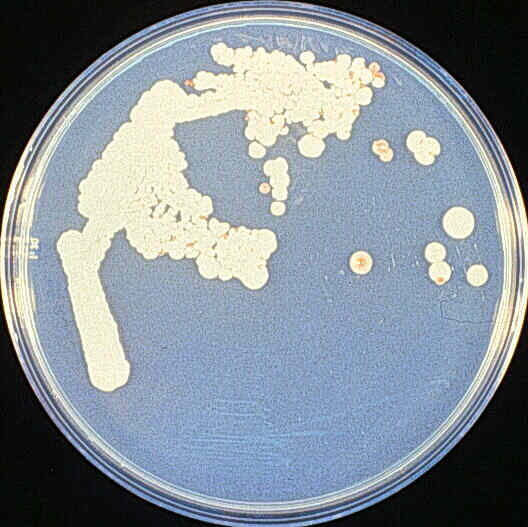 (The idea is that if done right, at some point on the solid media the “smearing” will have spread out the yeast cells far enough that you can make out the mounds of offspring that an individual yeast cell has made. Each distinguishable round spot of growth is effectively made up of millions of clones of the original single cell that started the “colony”)
(The idea is that if done right, at some point on the solid media the “smearing” will have spread out the yeast cells far enough that you can make out the mounds of offspring that an individual yeast cell has made. Each distinguishable round spot of growth is effectively made up of millions of clones of the original single cell that started the “colony”)
If everything works correctly, this gives you a “pure” culture, isolated from any other kinds of cells that may have been in the original sample. In this example, this is hopefully a brewing or bread yeast culture that you can now use to make beer, wine, bread, or fuel ethanol (the latter assuming you have permission from the Bureau of Alcohol, Tobacco, and Firearms, since it requires distillation.)
Tomorrow: Fun facts about yeast cultures.
 “Someone” seems to have located a replacement original disk of a game I had many years ago (but lost when I loaned it to
“Someone” seems to have located a replacement original disk of a game I had many years ago (but lost when I loaned it to 
 A pair of “barefoot shoes”
A pair of “barefoot shoes” Science is SATAN spelled backwards
Science is SATAN spelled backwards

 I get some odd Google searches hitting this site. Once in a while, however, I see one asking a question of vital importance and great usefulness to the general public. Today’s brief topic is this query: “Does beer and ice cream make gas?”.
I get some odd Google searches hitting this site. Once in a while, however, I see one asking a question of vital importance and great usefulness to the general public. Today’s brief topic is this query: “Does beer and ice cream make gas?”. +
+  =?????
=?????

 Wow! Look at that! Do I spy the
Wow! Look at that! Do I spy the 


 Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?
Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?




 What the heck? Is Green Dragon Spring actually devouring the ground under that walkway?!?! Oh! I get it – you’re saying the park itself can swallow you up and and cook you?
What the heck? Is Green Dragon Spring actually devouring the ground under that walkway?!?! Oh! I get it – you’re saying the park itself can swallow you up and and cook you?




