Here’s a brief interlude for my new readers. Members of my immediate family have seen this before elsewhere, but what the heck, I may as well share with you all.
Related to my recent posts about this year’s field-trip to Yellowstone National Park, I am reminded of a trip my wife and I took last year to Norris Geyser Basin in the same park.
I’ve heard of “communing with nature”, when the natural world seems to speak to you and put you at ease. Well, on this occasion it didn’t just seem to be speaking to me, and it hardly put me at ease. The conversation between me and the park went something like as follows that day. (Forgive all the scrolling, it WAS a several-hour conversation after all. If it bugs you, feel free to say so in the comments…)

Well, okay, I don’t smoke, I didn’t bring a bicycle, we left the dog at home. And I guess we can hold it until later. It’s a small price to pay to go out to nice, happy, peaceful trails, away from, for example, all the annoying road construction.

Wow! Look at that! Do I spy the
Fruiting Body of the rare Yellowstone Giant Orange Holewarning Mushroom?!?!?No, wait…that’s just a traffic cone over a hole in the trail! What the heck? I thought I was getting
away from road construction!
Well. Maybe things will get more natural-looking once we get a chance to walk off into the wilderness. Maybe the map’ll show where we can go.
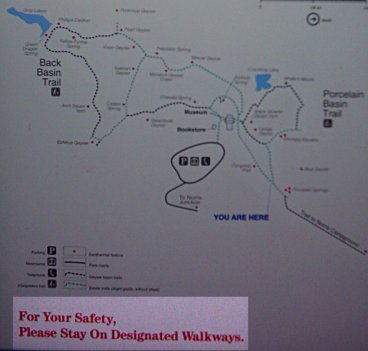
Ah, look at all the lovely trails.
Plenty of space to roam around in.
What? I can’t leave the trails?

Can’t I take a mere step or two off? Just a little bit???

 Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?
Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?

What kind of criminal act could possibly be involved with just leaving the silly walkway? Littering? Vandalism?

Oh, come on! Look, there’s a beautiful green pool over there, full of no doubt fascinating little animalcules. Can’t I just go over and take a little look?

“Thermal Area”? What the heck is that supposed to mean? It’s a little windy and chilly today, maybe I want to be warmed up a little. So, why not? What’s so bad about a “Thermal” area?

What’s that supposed to mean?

Children are prone to explode out of the ground unexpectedly?

What the heck? Is Green Dragon Spring actually devouring the ground under that walkway?!?! Oh! I get it – you’re saying the park itself can swallow you up and and cook you?
So everything here is flaming hot death, then, right?

Is there no end to the dangers this place threatens innocent visitors with? Can there possibly be anything else this park can do to us?!?!?

“Thin Crust”? “Boiling Water”? Broiled flesh cooked in acid? Ice?
Is this a park, or the kitchen at a Chinese restaurant? Is this how “Hot and Sour Soup” is made?!?!?
Is there nothing that can stand in this realm of violent burning chemical death without fear!?!?!? 
But what of us poor, fragile fleshy people? We aren’t safe here!That does it! I’m getting out of here! No more of these horrible Killer Thermal Death Areas of Doom for me! I think maybe I’ll just go look at the nice cuddly animals that Yellowstone is also famous for.

This whole friggin’ place wants to gore, dismember, maul, cook, devour, and digest me! That does it. No more of this dangerous stuff for me – I think I’ll just go read a nice book for a while…
 NOOOOOOOOOOOOOOOOOOOOOoooo!!!!!!
NOOOOOOOOOOOOOOOOOOOOOoooo!!!!!!








 Wow! Look at that! Do I spy the
Wow! Look at that! Do I spy the 


 Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?
Yeah, yeah, whatever. What are you going to do if I don’t – have me arrested?




 What the heck? Is Green Dragon Spring actually devouring the ground under that walkway?!?! Oh! I get it – you’re saying the park itself can swallow you up and and cook you?
What the heck? Is Green Dragon Spring actually devouring the ground under that walkway?!?! Oh! I get it – you’re saying the park itself can swallow you up and and cook you?










 Oh, right. Natural bottled-spring-water flavor. Hey, it’s natural, it’s got to be good for you, right?
Oh, right. Natural bottled-spring-water flavor. Hey, it’s natural, it’s got to be good for you, right?