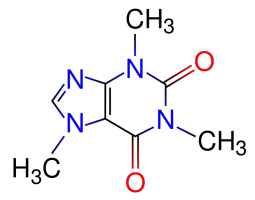
 Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
NO! Shenanigans! Caffeine is our FRIEND! And that stuff about it being a diuretic? CRAP! LIES AND SLANDER!
But don’t just take my word for it. After all, humans are a bunch of freakish multicellular soft-celled eukaryotes, and I normally focus on normal organisms like bacteria, archaea, and yeasts. So, let’s ask some real human-physiology type scientists and check out their official peer-reviewed findings:
Armstrong LE, Pumerantz AC, Roti MW, Judelson DA, Watson G, Dias JC, Sokmen B, Casa DJ, Maresh CM, Lieberman H, Kellogg M: “Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption.”; Int J Sport Nutr Exerc Metab. 2005 Jun;15(3):252-65.
“[…]The following variables were unaffected (P > 0.05) by different caffeine doses on days 1, 3, 6, 9, and 11 and were within normal clinical ranges: body mass, urine osmolality, urine specific gravity, urine color, 24-h urine volume, 24-h Na+ and K+ excretion, 24-h creatinine, blood urea nitrogen, serum Na+ and K+, serum osmolality, hematocrit, and total plasma protein. Therefore, C0, C3, and C6 exhibited no evidence of hypohydration.[…]”
Abstract on Pubmed
Armstrong LE, Casa DJ, Maresh CM, Ganio MS: “Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance.” Exerc Sport Sci Rev. 2007 Jul;35(3):135-40.
“[…]This review, contrary to popular beliefs, proposes that caffeine consumption does not result in the following: (a) water-electrolyte imbalances or hyperthermia and (b) reduced exercise-heat tolerance.”
(Review article, apparently – Abstract on Pubmed)
Del Coso J, Estevez E, Mora-Rodriguez R: “Caffeine effects on short-term performance during prolonged exercise in the heat.” Med Sci Sports Exerc. 2008 Apr;40(4):744-51.
“[…]RESULTS: Without fluid replacement (NF and NF + CAFF), subjects were dehydrated by 3.8 +/- 0.3%[…]CONCLUSION: During prolonged exercise in the heat, caffeine ingestion (6 mg.kg body weight) maintains MVC and increases PMAX despite dehydration and hyperthermia. When combined with water and carbohydrate, caffeine ingestion increases maximal leg force by increasing VA (i.e., reducing central fatigue).”
(“NF” = “No Fluid replacement” – the “dehydration” mentioned here is due to exercising in the heat, and doesn’t appear to be related to whether the test subjects consumed caffeine or not)
Abstract on Pubmed
Scott D, Rycroft JA, Aspen J, Chapman C, Brown B:”The effect of drinking tea at high altitude on hydration status and mood.” Eur J Appl Physiol. 2004 Apr;91(4):493-8. Epub 2004 Feb 11.
“[…]Several markers of hydration status were also taken immediately pre and post each condition, including measures of urine specific gravity, urine electrolyte balance (K+, Na+), and urine colour. None of these measures indicated a difference in hydration status as a result of the dietary intervention in either the control or tea condition.[…]”
(In this study, the tea was the only caffeine-containing substance involved. The study group’s caffeine came solely from the tea. The control group got no caffeine at all.)
Abstract on pubmed
Paluska SA: “Caffeine and exercise.” Curr Sports Med Rep. 2003 Aug;2(4):213-9.
“[…]It[caffeine] is relatively safe and has no known negative performance effects, nor does it cause significant dehydration or electrolyte imbalance during exercise.[…]”
Abstract on Pubmed
Grandjean AC, Reimers KJ, Bannick KE, Haven MC.: “The effect of caffeinated, non-caffeinated, caloric and non-caloric beverages on hydration.” J Am Coll Nutr. 2000 Oct;19(5):591-600.
“[…]This preliminary study found no significant differences in the effect of various combinations of beverages on hydration status of healthy adult males.[…]”
Pubmed entry – full text available
See? Oh, I know what you’re going to say next – “But, like, dude! When I drink my Venti Mocha Crappucino [note: Link goes to “Foamy the Squirrel”, who is a bit of a pottymouth, ranting about the “Tall/Grande/Venti” nonsense. It amused me.] or a can of Jolt Ultra I have to take a major whiz a little while later! Isn’t that ‘cuz of the caffeine?” Well, no, it isn’t. It’s because you just drank a bunch of liquid. Duh.
So, you see, caffeine really is our friend. Be nice to caffeine. But don’t feed it to your yeast in the presence of benzoic acid because it’ll kill them. See? I managed to turn this into a segue back to the stuff I was talking about before the whole “buy a house in Texas” thing started interfering. Stay tuned…



 Julius Robert Petri’s idea was so useful that we still use it today. Oh, yeah, and they named the
Julius Robert Petri’s idea was so useful that we still use it today. Oh, yeah, and they named the  To your right, you should see the ingredients used in this project. Yes, those two bottles in the background are there on purpose. I’m trying to make…Mountain Dew® Wine.
To your right, you should see the ingredients used in this project. Yes, those two bottles in the background are there on purpose. I’m trying to make…Mountain Dew® Wine.